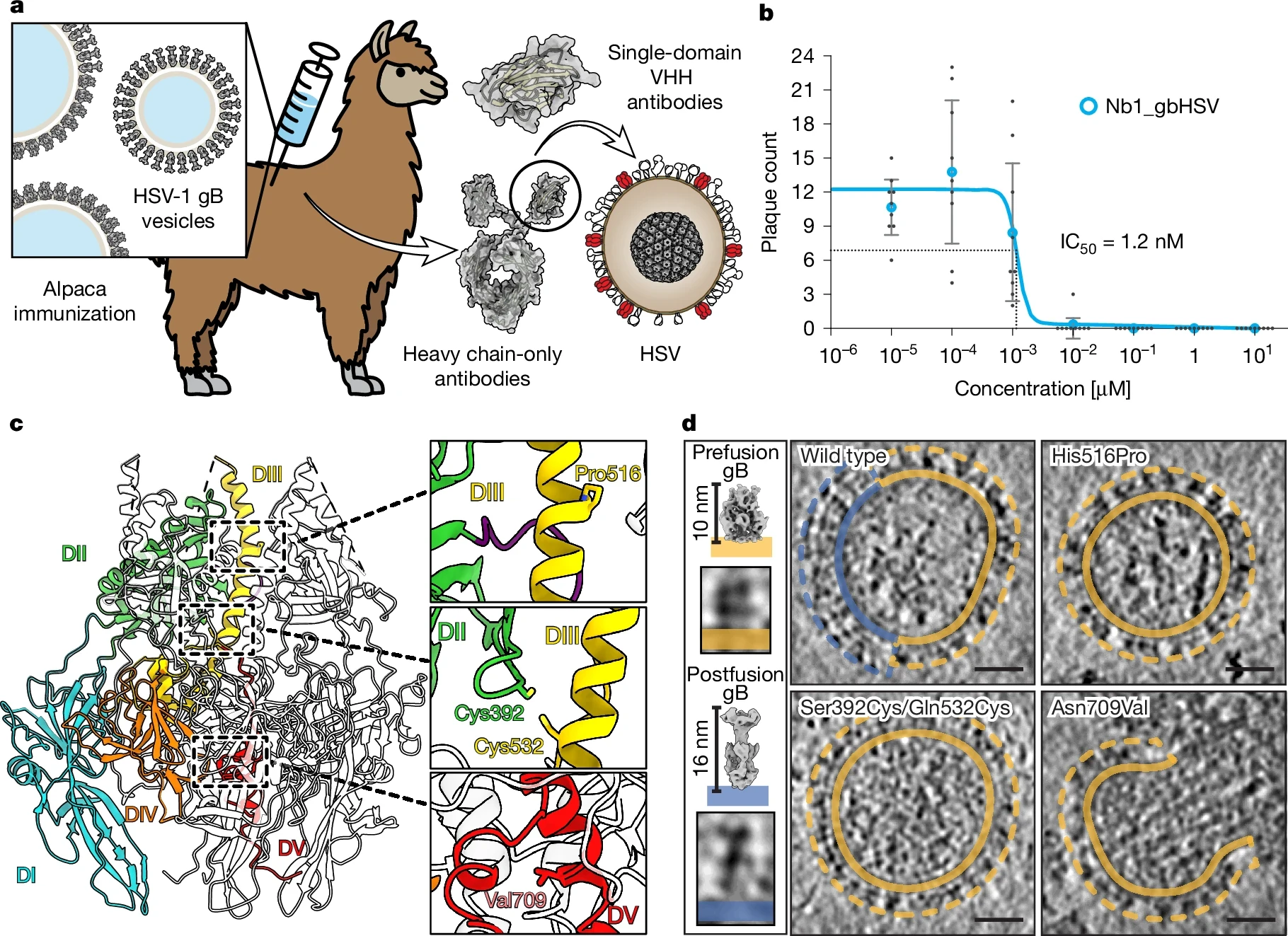
Recent research published in May 2025 highlights a groundbreaking new approach to cancer immunotherapy involving the herpes virus, specifically herpesvirus saimiri.
This virus naturally infects T cells in squirrel monkeys without causing disease and encodes proteins that activate critical signaling pathways inside T cells, notably the JAK-STAT5 pathway.
Activation of STAT5 enhances T cell survival, effector functions, and persistence—traits essential for effective anti-cancer immune responses.Scientists at the University of Michigan engineered a variant of a herpesvirus saimiri protein, the tyrosine kinase interacting protein (TIP), to directly activate STAT5 in T cells, bypassing the need for cytokines like interleukin-2 (IL-2), which are often scarce in the tumor microenvironment.
This engineered viral protein recruits kinases such as LCK in resting T cells to stimulate STAT5, sustaining T cell function even within the suppressive tumor milieu.
In mouse models of melanoma and lymphoma, this approach maintained T cell activity and significantly improved their ability to kill cancer cells, offering a promising new strategy to enhance T cell–based immunotherapies.
Herpesvirus Proteins May be the Key to Boost Cancer ImmunotherapyThis strategy represents a novel synthetic biology approach that repurposes viral mechanisms evolved to manipulate host cells, turning a pathogen’s tool into a therapeutic asset.
It may complement or enhance existing immunotherapies like CAR-T cell therapy, which can be hampered by the immunosuppressive environment tumors create.By directly activating intracellular signaling pathways, the engineered herpesvirus protein improves T cell durability and potency against cancer.
This approach bypasses the limitations of cytokine therapies, which often suffer from systemic toxicity and poor tumor penetration.Further research has combined oncolytic herpes simplex viruses engineered to express tumor antigens with CAR-T therapies, showing enhanced tumor cell killing and reprogramming of the tumor microenvironment to be more immunogenic.
This combination increased infiltration and activation of CD8+ T cells and dendritic cells, reduced regulatory T cells, and improved antigen presentation, collectively boosting anti-tumor immune responses in pancreatic cancer models.
Genetically modified herpes simplex viruses (HSVs) such as RP2 are under active clinical evaluation for advanced cancers including metastatic uveal melanoma and other solid tumors.
RP2 selectively infects and kills tumor cells while stimulating systemic anti-tumor immune responses.
It is currently in Phase 2/3 trials combined with immune checkpoint inhibitors like nivolumab, aiming to improve outcomes in patients resistant to standard immunotherapies.
Early Phase 1 data showed RP2 induced immune cell infiltration, notably CD8+ T cells, and activation of anti-cancer immune genes, with manageable side effects like fever and fatigue.MB-108, a second-generation HSV-1 oncolytic virus, received FDA orphan drug designation for malignant glioma treatment.
It is being tested in combination with CAR-T therapy (MB-101) to convert immunologically “cold” tumors into “hot” ones, enhancing CAR-T efficacy.
Phase 1 trials demonstrated safety and promising activity in recurrent glioblastoma.These next-generation HSV-based therapies incorporate genome engineering to improve tumor selectivity, safety, and immune stimulation.
Their combination with checkpoint inhibitors shows promise in overcoming resistance to immunotherapy, offering new hope for patients with refractory cancers.
Beyond direct oncolytic effects, herpesvirus proteins are being engineered to activate intracellular signaling pathways within T cells, particularly the JAK-STAT5 pathway, which is critical for T cell survival and function.
This approach bypasses the need for external cytokines like interleukin-2 (IL-2), which are often suppressed in the tumor microenvironment.By recruiting kinases such as LCK, these viral proteins sustain T cell effector functions even in hostile tumor environments, enhancing the durability and potency of immunotherapy.
This novel synthetic biology strategy leverages viral evolution to directly modulate immune cell signaling, offering a new avenue to overcome tumor-induced immunosuppression.
Next-Generation HSV-Based Oncolytic Immunotherapies
Recent reviews from 2023 to 2025 highlight significant progress in genome engineering of herpes simplex viruses (HSVs) to improve tumor selectivity, safety, and immune stimulation.
Next-generation oncolytic HSVs incorporate transgenes that boost immune activation and can be administered intratumorally or intravenously.Combining these engineered HSVs with checkpoint inhibitors or CAR-T therapies has shown promising results in preclinical and early clinical studies, particularly in tumors that are resistant to conventional immunotherapies.
These advances suggest that herpesvirus-based therapies could become vital components of future cancer treatment regimens.
Herpesvirus-based cancer immunotherapies represent an exciting frontier in oncology, harnessing the virus’s natural ability to manipulate immune signaling pathways to enhance T cell function and tumor destruction.With multiple candidates advancing through clinical trials and innovative viral protein engineering strategies emerging, the future looks promising for leveraging herpesviruses as powerful allies in the fight against cancer. Sources:
- An engineered viral protein activates STAT5 to prevent T cell suppression – PubMed
- An engineered viral protein activates STAT5 to prevent T cell suppression – Science Immunology
Bravado Labs Advanced Lysine Immune Boost
Why we love it:
- Verified Customer Favorite
- High Quality Ingredients
As an affiliate, we earn from qualifying purchases.
Recommended Supplements for Herpes Management
Simplix Viral Defense
Cold Sore & HSV Support
Simplix Viral Defense
Cold Sore & HSV Support
Synergistic formula combining L-Lysine, shiitake mushroom, and marine bioactives for comprehensive immune support.
SHOP NOW & SAVE 15%