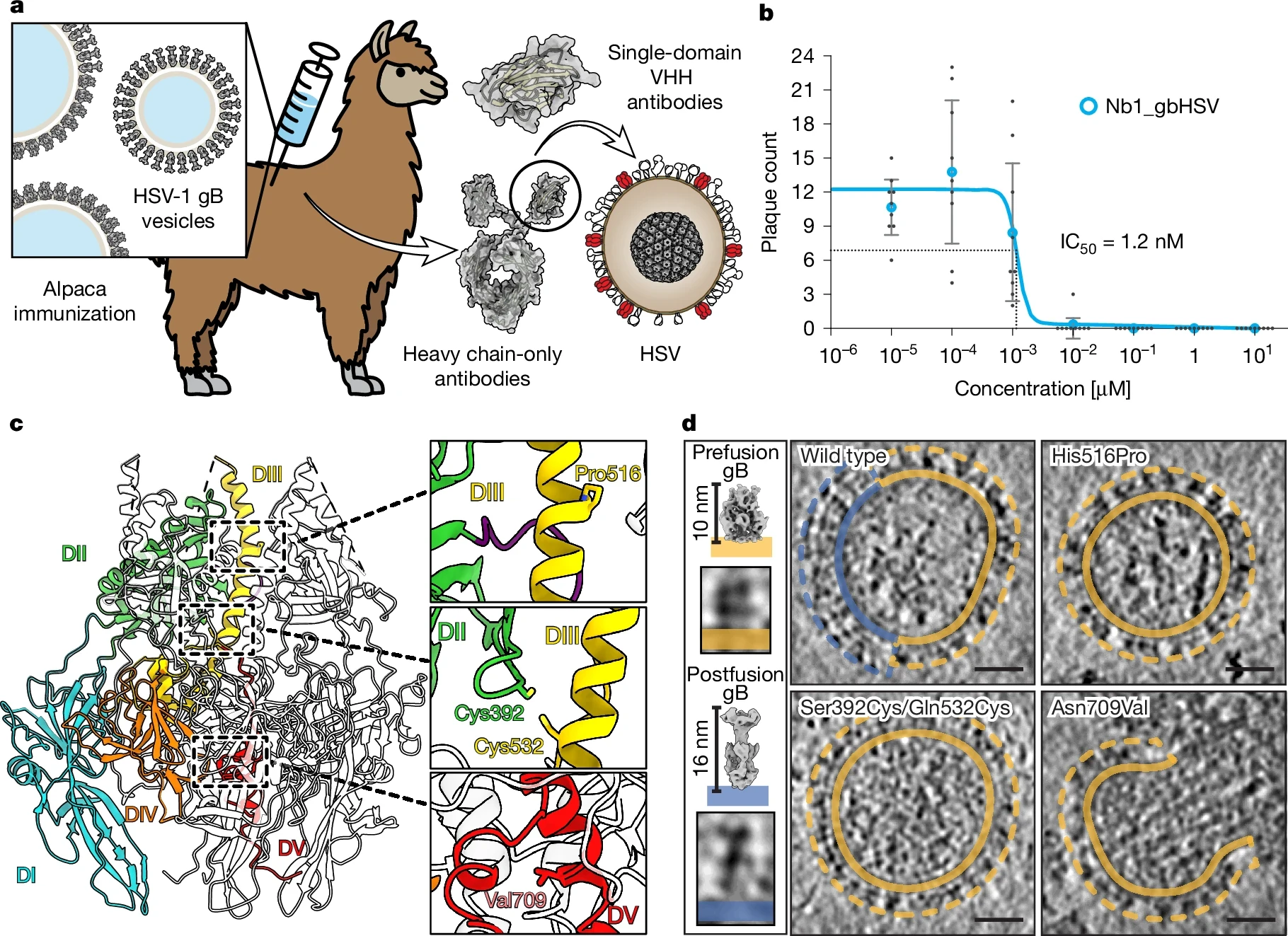
Herpes simplex virus (HSV) infections caused by HSV-1 and HSV-2 affect a significant portion of the global population, causing recurrent painful symptoms and imposing a substantial health and economic burden.
Despite decades of research, no vaccine has yet been approved to prevent or treat HSV infections.
However, recent advances in vaccine technology, particularly mRNA platforms, have spurred renewed efforts to develop effective vaccines.
This article reviews the current state of herpes vaccine research, highlighting leading candidates, ongoing clinical trials, and the realistic prospects for vaccine availability by 2026.
Challenges in Developing an Effective Herpes VaccineHSV’s ability to establish lifelong latent infections and evade the immune system through periodic reactivation complicates vaccine development.
This biological complexity has hindered past vaccine efforts.
Previous vaccine candidates, such as subunit vaccines GSK’s gD2 (Herpevac) and GEN-003, showed limited efficacy in preventing HSV-2 infection despite good safety profiles.
Live-attenuated vaccines elicit strong immune responses but raise safety concerns, especially in immunocompromised individuals.
Inactivated vaccines have been largely abandoned due to poor effectiveness.
Emerging DNA and viral vector vaccines show promise but face challenges in eliciting consistent human immune responses.
Leading Herpes Vaccine Candidates in Clinical Trials
mRNA Vaccines
Inspired by the success of COVID-19 mRNA vaccines, Moderna and BioNTech are developing mRNA-based HSV vaccines. Moderna’s mRNA-1608 targets HSV-2 and aims to induce robust antibody and cell-mediated immunity. Its Phase 1/2 clinical trial began in September 2023 and is expected to complete by June 2025. BioNTech’s BNT163 encodes three HSV-2 glycoproteins designed to block viral entry and counteract HSV-induced immunosuppression. Its Phase 1 trial started in December 2022, with results anticipated by December 2025. While promising, these vaccines’ clinical efficacy and safety in humans remain to be established.Live-Attenuated and Replication-Defective Vaccines
Rational Vaccines’ VC2, a live-attenuated HSV-1 vaccine candidate, has shown encouraging immune responses in animal models and may protect against HSV-2 genital herpes. It received $2.8 million NIH funding in late 2023 to advance clinical development. HSV529 (HSV15), a replication-defective virus vaccine developed by Sanofi Pasteur and the National Institute of Allergy and Infectious Diseases, is in early-phase trials. RVx201, another live-attenuated HSV-2 vaccine, is under observational clinical study in England.Other Therapeutic Approaches
Assembly Biosciences’ ABI-5366, a helicase primase inhibitor for recurrent genital herpes, is progressing through Phase 1 trials with good tolerability. Though not a vaccine, it represents an important therapeutic advance. Additional candidates include protein subunit vaccines, DNA vaccines, and novel adjuvant platforms such as NanoVax, all at various stages of development. Economic and Public Health ImportanceGenital herpes infections impose an estimated $35 billion annual economic burden worldwide due to healthcare costs and lost productivity.
Modeling suggests that an effective HSV vaccine could prevent up to 350,000 new genital herpes infections each year, significantly reducing this burden.
Beyond economics, a vaccine would improve quality of life by reducing painful recurrences and lowering transmission rates.
Realistic Timeline: Why a Licensed Herpes Vaccine by 2026 Is Unlikely
Despite the promising vaccine pipeline, experts agree that herpes vaccine availability by 2026 remains aspirational.
Most candidates, including Moderna’s mRNA-1608 and BioNTech’s BNT163, are still completing early to mid-stage clinical trials, with final data expected in 2025 or early 2026.
Following trial completion, vaccines require extensive data analysis, regulatory review, and manufacturing scale-up—processes that typically take several years.
Some candidates have faced setbacks, such as GSK’s recent discontinuation of its therapeutic HSV vaccine after failing Phase II efficacy endpoints.
Consequently, licensed herpes vaccines are more realistically expected to become available around 2030, reflecting the complexity of HSV biology and the rigorous standards for vaccine approval.
Summary and Outlook
Herpes simplex virus vaccine research is active and multifaceted, leveraging cutting-edge technologies including mRNA platforms, live-attenuated viruses, and replication-defective strains.
While no vaccine is currently approved, ongoing clinical trials are poised to provide critical data in the coming years.
Continued investment and research are essential to overcoming the challenges posed by HSV and ultimately delivering an effective vaccine that can reduce the global health burden of herpes infections.
Bravado Labs Advanced Lysine Immune Boost
Why we love it:
- Verified Customer Favorite
- High Quality Ingredients
As an affiliate, we earn from qualifying purchases.
Recommended Supplements for Herpes Management
Simplix Viral Defense
Cold Sore & HSV Support
Simplix Viral Defense
Cold Sore & HSV Support
Synergistic formula combining L-Lysine, shiitake mushroom, and marine bioactives for comprehensive immune support.
SHOP NOW & SAVE 15%