German researchers from institutions in Hamburg and Göttingen have achieved a major breakthrough in herpes research by developing a nanobody that can neutralize both HSV-1 and HSV-2.
The research, published in Nature on September 3, 2025, demonstrates how a tiny antibody derived from an alpaca can prevent herpes virus infection by targeting a critical viral protein.
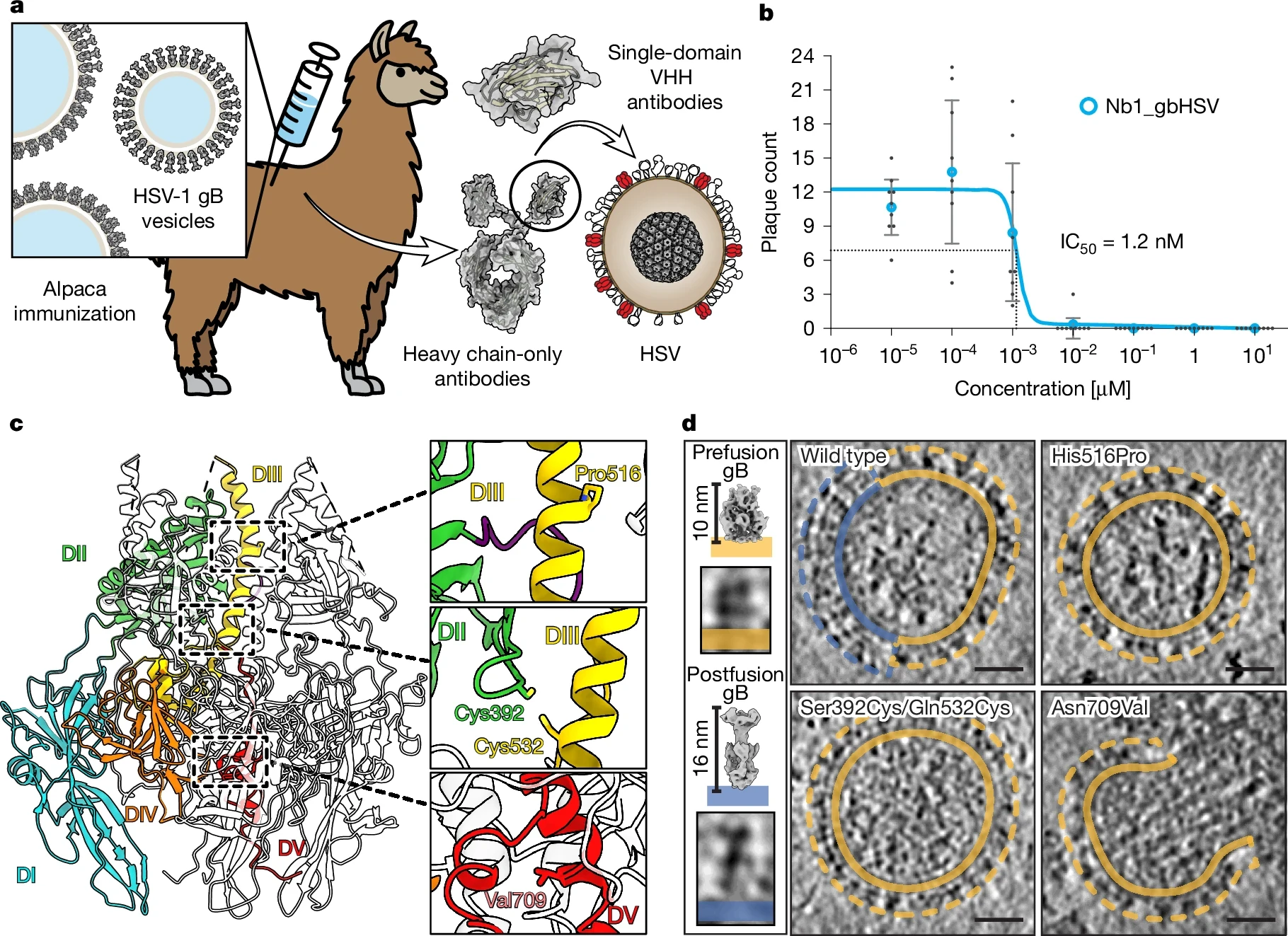
The international team, led by researchers from the Centre for Structural Systems Biology (CSSB) in Hamburg and the Max Planck Institute for Multidisciplinary Sciences in Göttingen, isolated the nanobody from an alpaca named Max that was immunized with the gB protein.
From approximately a billion different nanobodies, they identified one with exceptional neutralizing activity against both major types of herpes simplex virus.
How the Nanobody Works
The nanobody works by targeting glycoprotein B (gB), a crucial protein that herpes viruses use to infect cells.
To understand the significance of this discovery, it's important to know how herpes viruses operate during infection.
When a herpes virus infects a host cell, it first attaches to the cell membrane, then fuses its own membrane with that of the host cell.
This allows the virus to release its genetic material into the cell and begin replication.
The fusion process is driven by gB, which changes its three-dimensional shape to enable membrane fusion. The nanobody specifically binds to the prefusion form of gB, preventing it from performing the conformational changes required for fusion.
In essence, it freezes the virus's key fusion protein in place, stopping the infection process before it can begin.
Exceptional Binding Strength and Cross-Species Activity
What makes this nanobody particularly remarkable is its extraordinary binding affinity and cross-species effectiveness.
Microscale thermophoresis revealed a dissociation constant (KD) of approximately 14 picomolar for prefusion gB, while showing no measurable binding to the postfusion form.
This selective binding to the prefusion state is critical because viral fusion proteins often evade neutralization by rapidly transitioning to postfusion states. The nanobody works against both HSV-1 and HSV-2 because of the high sequence similarity in the targeted region between these two virus types.
Using cryo-electron microscopy, the Hamburg team successfully determined the 3D structure of HSV-2 gB bound to the nanobody, revealing insights into the neutralization mechanism and identifying critical binding sites.
Implications for Global Health
Approximately 60 percent of the global population carries HSV-1, which typically causes cold sores, while nearly 20 percent have genital herpes caused primarily by HSV-2, but also by HSV-1.
While herpes infections are often manageable for healthy individuals, they can have severe consequences for vulnerable populations.
Newborns are particularly at risk when mothers have active herpes infections during birth, as neonatal herpes can result in permanent neurological damage or death. People with weakened immune systems, including HIV-infected individuals, cancer patients, and organ transplant recipients, also face greater risks from herpes infections.
Current antiviral medications only work during active infections and do not prevent viral recurrence.
The nanobody approach offers potential benefits for both treatment and prevention of herpes infections.
Future Development and Challenges
While these results are promising, significant development work remains before clinical applications become possible.
The research team has filed patent applications to further develop the nanobodies for clinical use and attract industry partners for continued development.
Benjamin Vollmer, the lead scientist and first author of the study, noted that the nanobodies could potentially supplement existing medications and protect at-risk populations against herpes infection or recurrence of latent infections.
However, he emphasized that there is still a long way to go before these treatments reach patients.
The unique advantages of nanobodies, including their small size, stability, and ease of manufacturing compared to conventional antibodies, make them attractive candidates for therapeutic development.
Their ability to achieve higher tissue penetration could prove particularly valuable in treating herpes infections.
Bravado Labs Advanced Lysine Immune Boost
Why we love it:
- Verified Customer Favorite
- High Quality Ingredients
As an affiliate, we earn from qualifying purchases.
Recommended Supplements for Herpes Management
Simplix Viral Defense
Cold Sore & HSV Support
Simplix Viral Defense
Cold Sore & HSV Support
Synergistic formula combining L-Lysine, shiitake mushroom, and marine bioactives for comprehensive immune support.
SHOP NOW & SAVE 15%