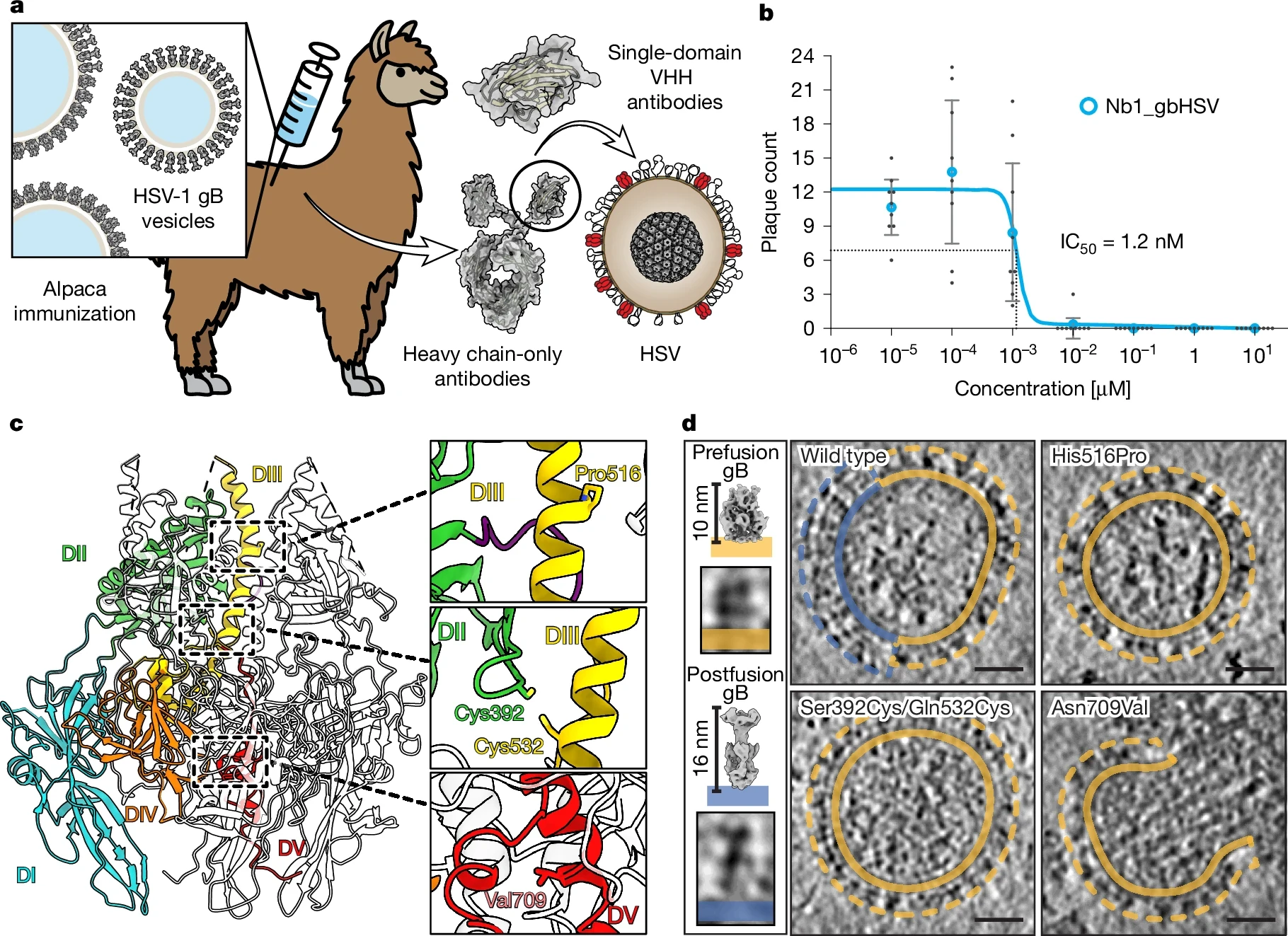
As we enter 2026, the global effort to develop an effective herpes simplex virus (HSV) vaccine has reached a critical turning point.
The landscape was significantly altered in late 2024 and throughout 2025 by the discontinuation of several protein-subunit candidates, most notably from GSK.
Consequently, the scientific community has consolidated its hopes around next-generation mRNA, live-attenuated platforms, and novel helicase-primase inhibitors.
Despite decades of research, no HSV vaccine has yet been approved for public use.
However, 2026 is viewed by experts as a "definitive data year." This article provides a detailed update on the most promising HSV vaccine candidates in 2026, highlighting their updated clinical statuses, the biological hurdles they must overcome, and what patients can realistically expect in the coming months.
Leading Vaccine and Therapeutic Candidates
mRNA-1608 is Moderna's premier therapeutic vaccine candidate designed specifically for individuals already living with recurrent genital herpes (HSV-2).
Unlike a preventative vaccine, mRNA-1608 functions as an "immunotherapy," training the host's immune system to better suppress viral activity.
The Phase 1/2 clinical trial (NCT06033261) progressed through 2025, reaching full enrollment.
As of early 2026, the study is analyzing primary endpoints: safety, immunogenicity, and the impact on "viral shedding." Viral shedding refers to the periods when the virus is active on the skin surface even without visible sores—a primary driver of transmission.
If mRNA-1608 can significantly reduce shedding days, it would represent a historic breakthrough in herpes management. 2026 Status: Researchers are currently evaluating the 12-month post-vaccination data.
This data will determine if Moderna moves into a large-scale Phase 3 pivotal trial by the end of 2026.
While the technology is promising, it is important to note that the vaccine is still not available for prescription or pharmacy purchase.
BioNTech's BNT163: Prophylactic Vaccine Progress
While Moderna focuses on those already infected, BioNTech's BNT163 is the lead candidate for prophylaxis (prevention).
The goal of BNT163 is to prevent HSV-2 infection in healthy, seronegative individuals.
It utilizes a "trivalent" approach, encoding three different HSV-2 glycoproteins (gC, gD, and gE) to block the virus's ability to enter cells and evade the immune system.
Throughout 2025, BioNTech's Phase 1 trials demonstrated that the vaccine was generally well-tolerated, with an immune profile similar to that of the COVID-19 mRNA vaccines.
In 2026, the focus has shifted to durability.
Scientists are measuring whether the antibody and T-cell responses remain high enough after 18 to 24 months to provide long-term protection against the virus.
If successful, BNT163 could become the foundation of a global effort to reduce the 11-13% global prevalence of HSV-2.
The "GSK Gap": Learning from Clinical Setbacks
A major development in the 2026 landscape is the absence of GSK's candidate (GSK3943104A).
GSK officially terminated this program in September 2024 after it failed to meet the required efficacy benchmarks in Phase 2 trials.
This was a significant blow to the community, as GSK's success with the Shingrix (Shingles) vaccine had sparked high hopes for a similar protein-subunit approach for HSV.
The failure of the GSK trial has led to a scientific consensus: traditional protein-subunit vaccines may lack the ability to stimulate the robust CD8+ T-cell response necessary to control a latent virus like herpes.
This "GSK Gap" has effectively ended the era of traditional subunit designs for HSV, forcing 2026 research to double down on mRNA and live-attenuated platforms that are better at mimicking a natural infection to provoke a stronger cellular immune response.
Assembly Biosciences: ABI-5366 and Helicase-Primase Inhibition
While not a "vaccine" in the traditional sense, ABI-5366 from Assembly Biosciences has become a major topic of interest in 2026.
This is a long-acting helicase-primase inhibitor designed for the treatment of recurrent genital herpes.
Unlike daily valacyclovir, which must be taken every 24 hours, ABI-5366 is being developed as a once-weekly or even once-monthly oral medication.
In 2026, Assembly Biosciences is moving into deeper Phase 1b/2 clinical trials.
The excitement surrounding this candidate stems from its potency; helicase-primase inhibitors block the virus's ability to unzip its DNA for replication more effectively than current nucleoside analogs.
For patients waiting for a vaccine, ABI-5366 represents a "bridge therapy" that could offer near-complete suppression of outbreaks and shedding until a permanent vaccine is approved.

Rational Vaccines: RVx201 and the Live-Attenuated Strategy
Rational Vaccines continues to champion the live-attenuated approach with their candidate RVx201.
This strategy uses a "live" but significantly weakened version of the HSV-2 virus.
Proponents argue that live-attenuated vaccines provide a much broader range of viral antigens to the immune system compared to mRNA or subunit vaccines.
As of 2026, the company is working under NIH support to refine its clinical program.
The primary hurdle for RVx201 remains safety validation.
Because the vaccine contains a live virus, regulatory agencies like the FDA require extensive proof that the vaccine strain cannot revert to a virulent state or establish harmful latency.
In 2026, the company is focusing on observational data and early-phase trials to prove that the "deletions" in the viral genome are stable and safe for human use.
Why the Timeline for 2026 is Critical
The year 2026 is critical because it marks the end of the "primary observation period" for the first wave of mRNA human trials.
Historically, many herpes vaccines have failed during the transition from Phase 2 to Phase 3.
The data released in 2026 will determine whether the billions of dollars invested in mRNA technology will finally result in a commercial product.
Additionally, the Herpes Cure Advocacy (HCA) and other patient groups have successfully pushed the NIH and FDA for a more streamlined regulatory pathway.
This means that if a candidate shows "breakthrough" results in 2026, there is a higher probability of accelerated approval or "Fast Track" status, which could shave years off the typical 10-year development cycle.
However, even with these accelerations, a vaccine is unlikely to be in pharmacies before 2028-2030.
The Concept of the "Functional Cure"
In 2026, the conversation has shifted from a "sterilizing cure" (completely removing the virus from the body) to a "functional cure." A functional cure refers to a treatment—likely a therapeutic vaccine like Moderna's mRNA-1608—that suppresses the virus so effectively that the patient:
- No longer experiences symptomatic outbreaks.
- Reduces viral shedding to near-zero levels.
- Eliminates the risk of transmission to partners.
Summary and Conclusion: The Outlook for 2026
While 2026 has not yet delivered a "silver bullet" approved vaccine, the narrowing of the field has led to more focused and sophisticated research. The industry has moved past the failed strategies of the 2010s and is now utilizing the most advanced genetic tools in medical history. The emphasis this year is on data transparency and Phase 3 readiness. The road to a herpes vaccine is paved with clinical setbacks, but the momentum in 2026 is undeniable. With mRNA leaders Moderna and BioNTech leading the charge and companies like Assembly Biosciences providing innovative antiviral alternatives, the community is closer to a solution than ever before. Continued global investment and participation in clinical trials remain the only path toward ending the global burden of HSV.
Herpes Vaccine Candidates 2026 FAQs
Is a herpes vaccine available to the public in 2026?
No. As of early 2026, all leading HSV vaccine candidates (including Moderna and BioNTech) are still in clinical trials. They are not yet available for purchase or prescription.What happened to the GSK herpes vaccine?
GSK terminated its HSV vaccine program (GSK3943104A) in late 2024. The Phase 2 data did not show enough efficacy to justify moving to Phase 3, leading the company to reallocate resources elsewhere.Is mRNA-1608 for HSV-1 or HSV-2?
Moderna's mRNA-1608 is specifically targeted at HSV-2 (genital herpes). However, because HSV-1 and HSV-2 share significant genetic similarity, researchers are monitoring participants for any "cross-protection" or benefits for HSV-1 (oral or genital).What is a "functional cure" for herpes?
A functional cure is a treatment that keeps the virus permanently suppressed. While the virus technically remains in the body's nerve cells, the person has zero symptoms and a negligible risk of transmitting the virus to others.How can I join a herpes vaccine trial in 2026?
Potential participants can search for active trials on ClinicalTrials.gov using keywords like "HSV vaccine" or "mRNA-1608." Many trials are still enrolling for long-term observational phases in 2026.Bravado Labs Advanced Lysine Immune Boost
Why we love it:
- Verified Customer Favorite
- High Quality Ingredients
As an affiliate, we earn from qualifying purchases.
Recommended Supplements for Herpes Management
Simplix Viral Defense
Cold Sore & HSV Support
Simplix Viral Defense
Cold Sore & HSV Support
Synergistic formula combining L-Lysine, shiitake mushroom, and marine bioactives for comprehensive immune support.
SHOP NOW & SAVE 15%