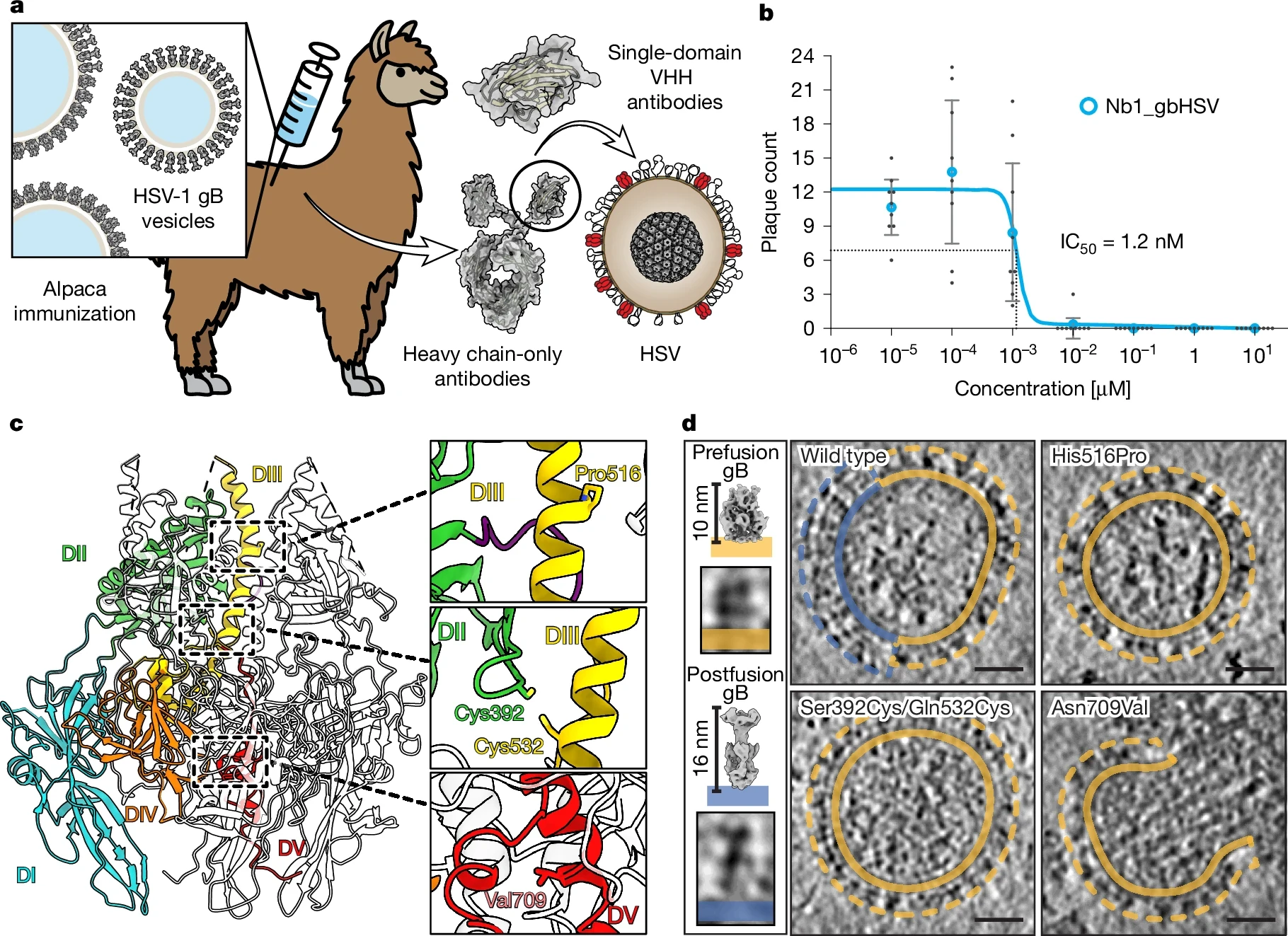
In 2025, the global effort to develop an effective herpes simplex virus (HSV) vaccine is gaining momentum with several promising candidates advancing through clinical trials.
HSV-1 and HSV-2 infections affect hundreds of millions worldwide, causing recurrent painful lesions, increasing susceptibility to other infections, and imposing a substantial economic burden.Despite decades of research, no HSV vaccine has yet been approved. However, recent breakthroughs in vaccine technology, especially mRNA platforms, have renewed optimism.
This article provides a detailed overview of the most promising HSV vaccine candidates in 2025, highlighting their mechanisms, trial progress, and potential impact on global health.
Moderna’s mRNA-1608: Therapeutic Vaccine for HSV-2
mRNA-1608 is Moderna’s innovative mRNA vaccine candidate designed to treat recurrent genital herpes caused by HSV-2.
It encodes viral glycoproteins that stimulate both neutralizing antibodies and cell-mediated immune responses, aiming to reduce outbreak frequency and severity.The vaccine is currently in a Phase 1/2 clinical trial initiated in September 2023, enrolling adults aged 18-55 with recurrent genital herpes.
The trial focuses on safety, dosing optimization, and immunogenicity, with results expected by mid-2025.
Key Highlights
- Targets multiple HSV-2 glycoproteins critical for viral entry
- Combines antibody and T-cell immunity for broad protection
- Potential cross-protection against HSV-1 is under study
Clinical Progress
The Phase 1/2 trial is ongoing, assessing safety and immune responses, with preliminary data anticipated in late 2025.Potential Impact
If successful, mRNA-1608 could become the first therapeutic vaccine to reduce HSV-2 recurrences and viral shedding, potentially lowering transmission and improving patient quality of life.Challenges
Demonstrating long-term durability of immune protection and efficacy across diverse populations remains a key hurdle.BioNTech’s BNT163: Prophylactic mRNA Vaccine Candidate
BNT163 is BioNTech’s prophylactic mRNA vaccine targeting HSV-2, encoding three viral glycoproteins essential for viral entry and immune evasion.
It aims to prevent infection before it occurs by priming the immune system.The vaccine entered Phase 1 clinical trials in late 2022, enrolling healthy adults without prior symptomatic genital herpes.
The trial evaluates safety, tolerability, and immunogenicity, with completion expected by December 2025.This candidate harnesses mRNA technology to induce a robust immune response that could prevent HSV-2 acquisition and reduce transmission.
Rational Vaccines’ RVx201: Live-Attenuated HSV-2 Vaccine
RVx201 is a live-attenuated HSV-2 vaccine developed by Rational Vaccines, with mutations in the ICP0 gene that reduce virulence while preserving immunogenicity.Preclinical studies demonstrated a 45% reduction in symptomatic days and fewer recurrent genital lesions in animal models.
An observational clinical study completed in 2023 assessed safety and immune responses in humans.Supported by a $2.8 million NIH grant, Rational Vaccines is advancing RVx201 and related candidates for both therapeutic and preventive uses against HSV-1 and HSV-2.
Delta gD-2: Live-Attenuated Vaccine Missing Glycoprotein D
Delta gD-2 is a live-attenuated HSV-2 vaccine candidate engineered by deleting glycoprotein D (gD), a protein essential for viral entry into host cells.This deletion renders the virus non-infectious but still capable of eliciting a strong immune response by exposing the immune system to attenuated viral antigens.While preclinical studies show promising protection, safety concerns remain for immunocompromised individuals due to the live virus platform.
Further clinical evaluation is needed to assess its suitability for widespread use.
HSV529: Replication-Defective Vaccine by Sanofi Pasteur and NIAID
HSV529 is a replication-defective HSV-2 vaccine developed by Sanofi Pasteur and the National Institute of Allergy and Infectious Diseases (NIAID).
It lacks two essential replication proteins, UL5 and UL29, preventing viral replication while still stimulating immune responses.Currently in Phase 1/2 clinical trials, HSV529 aims to combine the immunogenic advantages of live vaccines with an improved safety profile suitable for a broad population.This vaccine could provide effective protection without the risks associated with live-attenuated vaccines, representing a promising middle ground.
Conclusion: The Future of HSV Vaccines
The HSV vaccine landscape in 2025 is marked by innovative approaches including mRNA, live-attenuated, and replication-defective platforms.
Each candidate offers unique advantages and faces distinct challenges in the quest to prevent and treat herpes infections.Although no HSV vaccine has yet been approved, ongoing clinical trials are vital to establish safety and efficacy. The rapid advances in mRNA vaccine technology, spurred by COVID-19, have accelerated HSV vaccine research, raising hope for breakthroughs in the near future.Continued investment, rigorous clinical evaluation, and global collaboration will be essential to bring these promising vaccines to market and reduce the global burden of HSV infections.
Herpes Vaccine Candidates FAQs
What types of herpes vaccines are currently in development?
In 2025, herpes vaccine candidates include mRNA vaccines (like Moderna’s mRNA-1608 and BioNTech’s BNT163), live-attenuated vaccines (such as Rational Vaccines’ RVx201 and Delta gD-2), and replication-defective vaccines like HSV529. Each uses different strategies to stimulate immune protection against HSV-1 and HSV-2.
Are any herpes vaccines approved for use yet?
No herpes simplex virus vaccines have received regulatory approval as of 2025. All leading candidates are in various stages of clinical trials, with safety and efficacy data still being collected.
What is the difference between therapeutic and prophylactic herpes vaccines?
Therapeutic vaccines aim to reduce symptoms and viral shedding in people already infected with HSV, while prophylactic vaccines are designed to prevent infection in uninfected individuals.
How soon might a herpes vaccine become available?
While promising candidates are in late-stage trials, it may still take several years before an HSV vaccine is approved and widely available, depending on trial outcomes and regulatory review.
Can mRNA vaccine technology improve herpes vaccine development?
Yes, mRNA vaccine platforms have shown great potential to induce strong and targeted immune responses, accelerating herpes vaccine research and offering hope for effective vaccines against HSV.
Are live-attenuated herpes vaccines safe?
Live-attenuated vaccines can provide robust immunity but may pose safety concerns for immunocompromised individuals. Careful clinical evaluation is necessary to balance efficacy and safety.
How do replication-defective vaccines work?
Replication-defective vaccines use viruses engineered to be unable to replicate, reducing safety risks while still eliciting immune responses similar to live vaccines.
What challenges remain in developing an effective herpes vaccine?
Key challenges include targeting latent virus reservoirs, overcoming HSV’s immune evasion mechanisms, ensuring long-lasting immunity, and demonstrating efficacy in diverse populations.
Bravado Labs Advanced Lysine Immune Boost
Why we love it:
- Verified Customer Favorite
- High Quality Ingredients
As an affiliate, we earn from qualifying purchases.
Recommended Supplements for Herpes Management
Simplix Viral Defense
Cold Sore & HSV Support
Simplix Viral Defense
Cold Sore & HSV Support
Synergistic formula combining L-Lysine, shiitake mushroom, and marine bioactives for comprehensive immune support.
SHOP NOW & SAVE 15%