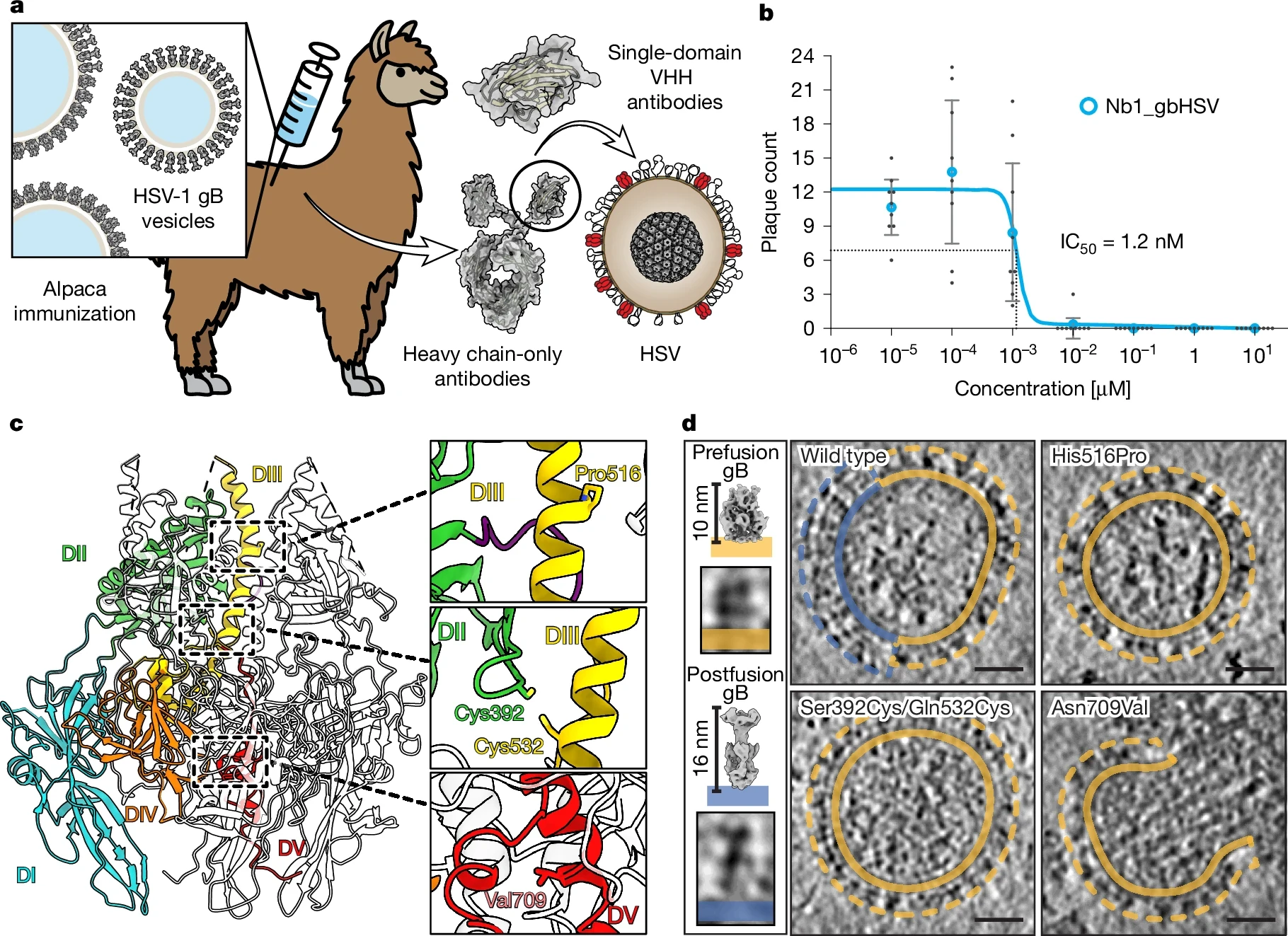
Herpes simplex virus (HSV) infections caused by HSV-1 and HSV-2 affect approximately 3.7 billion people globally—roughly 67% for HSV-1 and 11% for HSV-2—causing recurrent painful symptoms and imposing a substantial health and economic burden.
Despite decades of research, no vaccine has yet been approved to prevent or treat HSV infections.
However, recent advances in vaccine technology, particularly mRNA platforms proven successful in COVID-19 development, have spurred renewed efforts to develop effective vaccines.
This article reviews the current state of herpes vaccine research as of late 2025, highlighting leading candidates, ongoing clinical trials, the biological and regulatory barriers to development, and the realistic prospects for vaccine availability beyond 2026.
Challenges in Developing an Effective Herpes Vaccine
HSV's ability to establish lifelong latent infections in nerve cells and evade the immune system through sophisticated viral mechanisms complicates vaccine development.
Unlike acute viral infections like measles or polio, HSV cannot be completely cleared from the body, requiring a vaccine that prevents infection or reduces recurrence severity—a substantially higher bar than traditional vaccines. Immune evasion strategies: HSV has evolved multiple mechanisms to avoid immune destruction, including downregulating MHC molecules on infected cells, producing viral IL-10 homologs that suppress interferon responses, and interfering with natural killer cell activation.
These mechanisms mean that antibody-mediated immunity alone may be insufficient for protection. Historical vaccine failures: Previous vaccine candidates showed the difficulty of overcoming these challenges.
GSK's Herpevac (gD2 subunit vaccine with AS04 adjuvant) achieved 73% efficacy against HSV-1 in seronegative women but only 20% against HSV-2, and offered no benefit to people already infected with HSV-1.
Genocea's GEN-003 therapeutic vaccine failed to demonstrate efficacy in reducing recurrence rates in Phase II trials.
These failures suggest that simple subunit approaches are insufficient. Platform tradeoffs: Live-attenuated vaccines elicit stronger T-cell and mucosal immune responses but raise safety concerns, especially for immunocompromised populations.
Inactivated vaccines have shown poor effectiveness.
DNA and viral vector vaccines show promise in animal models but have struggled to generate consistent protective immunity in humans.
Leading Herpes Vaccine Candidates in Clinical Trials
Following the success of COVID-19 mRNA vaccines, both Moderna and BioNTech are leveraging lipid nanoparticle technology to deliver HSV immunogens.
However, experts caution that HSV's biological complexity differs fundamentally from SARS-CoV-2, and vaccine effectiveness cannot be assumed simply because the platform succeeded previously. Moderna's mRNA-1608: This HSV-2 vaccine candidate consists of mRNA sequences encoding HSV-2 glycoproteins packaged in lipid nanoparticles.
The Phase 1/2 clinical trial began in September 2023 and was initially expected to complete by June 2025.
However, as of December 2025, trial completion has been extended into early 2026 to allow for longer safety and immunogenicity follow-up.
The trial evaluates antibody responses, T-cell immunity (CD4+ and CD8+), and preliminary protection signals.
Full efficacy data requiring Phase 3 enrollment are not expected until 2027-2028 at the earliest.
Moderna has publicly stated that regulatory approval by 2026 is not feasible. BioNTech's BNT163: This HSV-2 vaccine encodes three viral glycoproteins (gG, gC, and gE) designed to block multiple viral entry pathways and counteract HSV-induced immunosuppression.
Phase 1 trials began in December 2022, with interim immunogenicity data presented at medical conferences in 2024 showing strong CD8+ T-cell responses.
Phase 2 enrollment is expected to initiate in mid-2026, with efficacy readouts unlikely before 2027-2028.
Like Moderna, BioNTech has indicated that 2026 approval is not a realistic target. Live-Attenuated and Replication-Defective Vaccines: Rational Vaccines' VC2 is a live-attenuated HSV-1 vaccine containing deletions in genes responsible for immune evasion (US3, US5) and latency establishment (ICP0), designed to elicit robust cellular immunity while reducing neurovirulence.
Animal studies show encouraging CD8+ T-cell responses and partial protection against lethal HSV-2 challenge.
Rational Vaccines received \$2.8 million in NIH funding in late 2023 to support IND-enabling studies.
Clinical Phase 1 trials are expected to initiate in 2026, making efficacy data unlikely before 2028-2029.
HSV529 (HSV15), a replication-defective HSV-1 vaccine developed by Sanofi Pasteur and NIAID, contains deletions in helicase-primase genes (UL5, UL29) and cannot replicate in most cell types but retains immunogenicity.
Early Phase 1 data (2023-2024) demonstrated acceptable tolerability and T-cell immunogenicity.
Phase 1b/2a expansion studies were ongoing as of late 2025, with full Phase 2 enrollment anticipated for 2026.
RVx201, another HSV-2 live-attenuated vaccine candidate, is currently under observational clinical study in England with limited publicly available data.
Genocea's GEN-003, though it failed earlier efficacy trials, is being investigated through alternative pathways with enhanced adjuvants. Complementary Therapeutic Approaches: Assembly Biosciences' ABI-5366, a helicase-primase inhibitor, represents an important complementary therapeutic approach for reducing recurrent genital herpes outbreaks.
Phase 1 trials demonstrated favorable tolerability, with Phase 2 efficacy studies expected to begin in 2026.
Combined strategies using antivirals plus vaccines may emerge as the optimal treatment paradigm.
Additional candidates in preclinical or early Phase 1 stages include protein subunit vaccines with novel TLR agonist adjuvants, DNA vaccines with optimized codon usage and immunostimulatory sequences, viral vector-based vaccines using modified vaccinia or adenovirus platforms, and mucosal vaccine formulations designed to induce local immunity at primary infection sites.
Economic and Public Health Importance
The burden of HSV infections extends far beyond personal suffering.
Genital herpes alone imposes estimated direct medical costs of \$1,600-\$2,400 per patient annually in the United States, with global economic burden estimated at \$30-40 billion annually when including lost productivity, absenteeism, psychological impacts, and neonatal herpes complications. Prevention potential: Mathematical modeling conducted by public health researchers suggests that an effective HSV vaccine achieving 70-80% efficacy could prevent approximately 350,000-500,000 new genital HSV-2 infections annually in the United States alone.
At a global scale, this represents millions of preventable infections and substantial economic savings across healthcare systems. Quality of life impact: Beyond economics, an effective vaccine would dramatically improve quality of life by reducing recurrent painful outbreaks (which average 4-5 episodes annually for symptomatic individuals), decreasing psychological burden and depression associated with chronic herpes, reducing sexual transmission anxiety, and preventing neonatal herpes transmission when mothers are vaccinated before pregnancy.
Approximately 350-500 cases of neonatal herpes occur annually in the United States alone, often causing severe neurological sequelae or death. Asymptomatic transmission challenge: An estimated 50-60% of primary genital HSV-2 infections are asymptomatic or minimally symptomatic, making silent transmission a significant epidemiological driver.
A vaccine reducing viral shedding in asymptomatic individuals could have population-level effects exceeding individual-level protection.
Why a Licensed Herpes Vaccine by 2026 Is Unlikely: Regulatory and Development Barriers
Despite promising candidate pipelines, the consensus among vaccine developers, regulatory scientists, and HSV immunologists is clear: herpes vaccine availability by 2026 is highly unlikely.
Several interconnected factors explain this realistic assessment. Current trial phase status: As of December 2025, most leading candidates remain in Phase 1 or Phase 1/2 trials.
Moderna's mRNA-1608 and BioNTech's BNT163 are completing Phase 1/2 immunogenicity studies with efficacy data not expected until 2027-2028.
VC2 has not yet entered clinical trials.
HSV529 is in early Phase 2.
These timelines mean that candidates will not be ready for Phase 3 efficacy trials—the critical studies required for regulatory approval—until 2026-2027 at the earliest. Phase 3 trial requirements and duration: Pivotal Phase 3 efficacy trials for a preventive herpes vaccine typically require 3,000-6,000 participants followed for 12-24 months to capture adequate infection events.
These large, long-term studies typically require 2-4 years to complete, recruiting participants, following them longitudinally, monitoring infections, and collecting comprehensive safety data.
Most HSV vaccine Phase 3 trials would not complete before 2028-2030. Regulatory review timeline: Following Phase 3 completion, regulatory agencies (FDA, EMA) require 12-24 months of standard review time for Biologics License Applications.
Even with Priority Review designation (6 months), approval would not occur before 2029-2030 for candidates just entering Phase 3 in 2026-2027. Manufacturing and scale-up: Successful trial results must be followed by manufacturing process optimization, GMP compliance validation, stability studies (typically 18-24 months minimum), and commercial-scale production.
This process alone typically requires 2-3 years post-approval. Recent program setbacks: GSK's December 2024 discontinuation of its therapeutic HSV vaccine program following Phase II efficacy failure serves as a cautionary reminder.
Despite strong safety data, the vaccine failed to meet efficacy thresholds for reducing recurrence burden in HSV-2 seropositive patients.
This setback demonstrates that advancing to approval requires not just safety but also compelling efficacy signals. Expert consensus on realistic timelines: In interviews and public statements (2024-2025), executives from Moderna, BioNTech, and Sanofi have explicitly stated that 2030-2032 is a more realistic timeframe for herpes vaccine availability, reflecting the complexity of HSV immunology, the rigor required for vaccine approval, and the need for adequate long-term safety surveillance.
Regulatory Acceleration Pathways and Development Factors
Breakthrough Therapy Designation: Should Phase 2 data demonstrate unexpected efficacy signals substantially exceeding historical vaccine standards, the FDA could grant Breakthrough Therapy Designation, potentially accelerating timeline by 12-18 months through priority review and expedited regulatory interactions. However, no HSV vaccine candidate has achieved this designation, and breakthrough designation requires demonstration of meaningful clinical benefit—a high bar given that previous vaccine attempts have failed efficacy endpoints. Conditional/Provisional Approval Pathways: European and some international regulatory agencies offer conditional approval mechanisms for vaccines addressing high unmet medical needs. These could theoretically enable limited availability in specific populations (e.g., high-risk individuals) while Phase 3 trials continue. However, such pathways remain rarely used and typically require extraordinary circumstances or severe public health crises. Factors That Could Accelerate Development:
- Unexpectedly strong immunogenicity or preliminary efficacy signals in Phase 1/2 trials
- Expanded funding from government agencies or philanthropic organizations accelerating enrollment
- International regulatory harmonization reducing redundant trial requirements across jurisdictions
- Discovery of biomarkers enabling shorter trial durations or enrichment strategies
- Combination strategies (vaccine plus antivirals) demonstrating additive benefit enabling faster approval
Factors Likely to Extend Development:
- Inadequate immunogenicity or preliminary efficacy signals requiring trial redesign
- Unexpected safety signals necessitating additional monitoring or dose adjustments
- Manufacturing challenges delaying scale-up (precedent: vaccine shortages for other pathogens)
- Regulatory requests for additional data (particularly common for novel platforms)
- Difficulty recruiting and retaining trial participants over extended follow-up periods
- HSV reactivation or latency-related adverse events in vaccine recipients
Comparative Timeline: How Herpes Vaccine Development Compares to Other Vaccines
Understanding the historical timeline for vaccine development provides context for realistic herpes vaccine expectations: COVID-19 vaccines (mRNA): ~12-14 months from sequences to emergency use authorization.
However, this unprecedented speed benefited from massive funding (\$10+ billion), existing mRNA platform infrastructure, population-wide testing for efficacy readouts, global regulatory cooperation, and acceptance of rare adverse event risks.
Herpes vaccine development cannot replicate these conditions. HPV vaccines (Gardasil, Cervarix): ~15 years from discovery to approval (1991-2006).
This timeline included basic immunology research, preclinical studies, Phase 1-3 trials, and manufacturing development.
HPV vaccines also benefited from clearer immunological mechanisms of protection than HSV. Rotavirus vaccines: ~13-15 years from discovery to approval.
Multiple candidate failures and manufacturing complications extended timelines. Shingles vaccine (Shingrix): Over 20 years from initial research to final approval as an improved recombinant vaccine, replacing earlier live-attenuated versions.
Given this historical context and HSV's greater biological complexity compared to most vaccine targets, a 7-10 year development timeline (2025-2032) is realistically aligned with precedent.
Summary and Realistic Outlook
Herpes simplex virus vaccine research has genuinely accelerated, leveraging cutting-edge platforms including mRNA technology, live-attenuated viruses with enhanced safety profiles, and replication-defective strains.
The current pipeline is diverse and robust, with at least 4-5 serious candidates in clinical development and numerous others in preclinical stages. However, the realistic assessment is unambiguous: a licensed herpes vaccine by 2026 is highly unlikely. Most leading candidates will still be in Phase 1/2 trials through 2025-2026, with Phase 2/3 efficacy trials extending into 2027-2029, regulatory review requiring 2027-2030, and commercial availability likely around 2030-2032. The genuine good news: This timeline represents substantial acceleration compared to historical vaccine development and reflects real scientific progress.
Candidate vaccines exist.
Clinical trials are underway.
Regulatory pathways are in place.
Investment is committed.
While 2026 will disappoint those hoping for imminent availability, it is realistic to expect meaningful efficacy data by 2027-2028, regulatory approval by 2029-2031, and herpes vaccine availability in clinical practice by 2031-2033.
For the approximately 3.7 billion people living with HSV infection, an effective vaccine within the next 5-7 years represents a profoundly meaningful health advance.
Continued investment, sustained research funding, and international collaboration remain essential to realizing this goal and ultimately reducing the global health burden of herpes infections. Key Takeaway for Patients and Healthcare Providers: While herpes vaccines are not available in 2026, they remain on the near-term horizon.
Individuals seeking prevention can continue utilizing existing strategies (condoms, antivirals for serodiscordant couples, suppressive therapy for frequent recurrence).
Healthcare providers should remain informed about clinical trial availability for patients interested in participating, as trial participation may accelerate vaccine development timelines.
Bravado Labs Advanced Lysine Immune Boost
Why we love it:
- Verified Customer Favorite
- High Quality Ingredients
As an affiliate, we earn from qualifying purchases.
Recommended Supplements for Herpes Management
Simplix Viral Defense
Cold Sore & HSV Support
Simplix Viral Defense
Cold Sore & HSV Support
Synergistic formula combining L-Lysine, shiitake mushroom, and marine bioactives for comprehensive immune support.
SHOP NOW & SAVE 15%